Microbial Genomics: Standing on the Shoulders of Giants
Professor Sir David Hopwood, part I

Sixty years of Streptomyces genetics: from Petri dish to computer
From graduating with a degree in botany at Cambridge University, to a PhD in microbial genetics, to co-ordinating the sequencing of the genome of Streptomyces coelicolor, the largest microbial genome to be sequenced at the time, David Hopwood has had a momentous and fascinating career interacting with many great scientists and promoting the development of microbial genomics.
Part I – First steps towards Streptomyces genetics
In 1954 I had graduated in botany at Cambridge and was looking for an interesting PhD project. Harold Whitehouse, who taught genetics in the Botany School, had pointed out that microorganisms were on the threshold of a great future as the centre of efforts to elucidate the nature and mode of action of genes because of their rapid life cycles and huge population sizes so I wanted to be part of this exciting prospect. Fungal genetics, notably the seminal work of George Beadle and Edward Tatum with Neurospora, whose studies of auxotrophic mutants led to the 'one gene-one enzyme' hypothesis, had been based on the known life cycle of the fungus with its strict alternation of haploid and diploid phases separated by a meiosis in which complete genomes of two parents were recombined and re-assorted in a regular and predictable way. This is the almost universal pattern in what came to be known as the eukaryotic kingdom, which includes plants, animals and people. But bacterial genetics was a confusing medley of at least three processes, none of which fitted the eukaryotic paradigm. There was transformation in the pneumococcus, in which fragments of DNA from a donor strain were taken up by a 'recipient' and inherited by recombinant offspring. In transduction in Salmonella, pieces of the genome of a donor bacterium were transferred to a recipient by incorporation into phage particles. Conjugation in Escherichia coli. came nearest to eukaryotic sex because contact between two organisms was required for DNA transfer, but it was a bizarre process of gradual and incomplete mating. Three processes, all different and all contrasting with the eukaryotic nuclear fusion and chromosome segregation model.
It was against this background that Lewis Frost, the other geneticist on the faculty of the Botany School, suggested that I work on Streptomyces. They were the best known members of the actinomycetes, a group of microbes discovered towards the end of the nineteenth century, which were usually regarded as intermediate between bacteria and fungi. They grew as a mycelium, a system of interconnected, branching hyphae characteristic of fungi, but their cellular dimensions were those of typical bacteria. Nothing substantial was known about their genetics so, if they really were a missing link between the two known kingdoms of life (the archaea were still to be defined), what strange phenomena might await discovery? I accepted the challenge and set to work on some Streptomyces cultures that Lewis Frost had already acquired.
I settled on one of the strains, which grew well and made a beautiful blue pigment which I knew from the literature was characteristic of Streptomyces coelicolor. I thought that the blue colour might eventually make a useful genetic marker, not realising then that, being an antibiotic, it would offer a great tool for analysing the genetics of antibiotic biosynthesis. Following in the footsteps of Joshua Lederberg who, working with Tatum, had discovered genetic recombination in E. coli by mixing pairs of auxotrophic mutants and selecting rare prototrophic recombinants from amongst millions of parental genotypes, I isolated auxotrophs using the Lederbergs’ replica plating technique and showed that recombination did indeed occur at a low frequency. A critical finding was that, in mixtures of strains with more than one marker, some of the recombinants inherited presumed recessive characters (auxotrophy rather than prototrophy) from both parents, demonstrating a true recombination process rather than merely a, perhaps transitory, association of two parental organisms. This also suggested that large segments, at least, of the genomes were transferred, indicating a kind of conjugation rather than a transformation or transduction process. I was quite pleased with myself when I went on to devise a means of analysing four-factor crosses to build a preliminary linkage map for the organism.
Meanwhile, the ripeness of the actinomycetes for genetic study had not gone unnoticed and several others around the world had started experiments unknown to each other. Notable were Giuseppe Sermonti and his wife Isabella Spada-Sermonti at the Istituto Superiore di Sanità in Rome. They actually published ahead of me, using another strain of S. coelicolor, and drew similar conclusions. However, they had not reached the stage of genetic mapping so I shared my strains with them and spent a couple of wonderful sojourns in Rome in 1960 and 1961 having a great time and doing collaborative experiments before they, and the others who had begun work on Streptomyces genetics, moved on to other topics.
Not long into my PhD studies I had the great good fortune to meet Audrey Glauert, a brilliant electron microscopist at the Strangeways Laboratory in Cambridge who was pioneering thin sectioning of bacteria to reveal their internal structures. We had a great collaboration during which we saw that S. coelicolor had a typical bacterial anatomy with no nuclear envelope separating the DNA from the cytoplasm with its densely packed ribosomes (Figure 1). In the words of Bill Hayes, one of the pioneers of E. coli genetics, when he chaired a session of the Genetical Society at which I presented our results, the organisms were ‘senior bacteria’, not ‘junior fungi’, nor intermediate organisms. This did not reduce my desire to carry on studying them because they were clearly phylogenetically far from the simple rod-shaped bacteria that had been studied and so were likely to reveal genetic novelty.
During my mapping work I often isolated recombinant strains from one cross and used them as parents in subsequent crosses as I added more and more markers to the linkage map. It soon became apparent that such crosses tended to yield recombinants at much higher frequency than the first crosses that used mutants isolated directly from the wild-type strain. It was all quite confusing but largely through the painstaking work of post-doc Alan Vivian and my long-term assistant and eventual colleague Helen Ferguson (later Helen Wright and finally Helen Kieser) it was possible to divide the strains into fertility types reminiscent of those due to the various states of the F factor in E. coli. We invoked a putative plasmid with autonomous or integrated possibilities, or absence from the strain, to account for the different levels of recombinant production in crosses. This hypothetical element fulfilled Lederberg’s definition of a plasmid as an extrachromosomal genetic element, irrespective of its physical structure, but we could not isolate it physically by any published method for extracting covalently closed circular (CCC) DNA molecules, the form in which all then known plasmids existed. We took a bit of flak for using the term plasmid for SCP1 in the absence of its physical characterisation but stuck to our guns and were eventually vindicated when Haruyasu Kinashi showed it to be a linear molecule that could be visualised only by the later developed method of pulsed-field gel electrophoresis. Meanwhile, Hildgund Schrempf isolated a normal CCC plasmid from our strains and this came to be called SCP2. Mervyn Bibb took up its study and showed it also to be a sex factor. Its great importance, however, was that it could be physically isolated and so eventually could be developed as a vector for gene cloning.
Another key development at this time was the isolation and study of phages for S. coelicolor, first from soil by my PhD student John Dowding and later by a scientist who became a great friend and collaborator, Natalia Lomovskaya at the Institute for the Genetics of Industrial Microorganisms in Moscow. Her group isolated a temperate phage they called phiC31 which my colleague Keith Chater developed into wonderfully versatile cloning vectors for S. coelicolor.
Before we leave the topic of natural genetic recombination promoted by sex plasmids for the sunny uplands of artificial genetic manipulation it is worth remembering that mutant isolation and genetic mapping were crucial in beginning the study of the two main characteristics that set the streptomycetes apart from most other bacteria. The first is the 'multicellular' life-style, from dormant spore to vegetative or 'feeding' mycelium, then to the reproductive aerial mycelium as branches from the vegetative mycelium and back to spores (Figure 2). The second is the unrivalled ability of the streptomycetes to make antibiotics. Helen and I had started to isolate and map mutants blocked at two critical points in the life cycle – the origin of the aerial mycelium, revealed by the 'bald' (bld) mutants, and the segmentation of the aerial mycelium into spores, revealed by the 'white' (whi) mutants that fail to develop into the grey-pigmented spores. Study of these classes of mutants became the career-long topic of Keith Chater’s research as he and others developed S. coelicolor into a key model of bacterial developmental biology. In parallel, PhD students Ralph Kirby, Fred Wright and Brian Rudd laid the groundwork for the field of antibiotic genetics, discovering that the SCP1 plasmid carries genes for the biosynthesis of a colourless antibiotic, methylenomycin, while two pigmented antibiotics – the blue actinorhodin and the red undecylprodigiosin – are controlled by clusters of chromosomal genes (Figure 3).
This is the end of the first part of my story in which natural means of gene exchange in Streptomyces were discovered and harnessed to study the genetics of the organism. Next time we move to artificial means of achieving gene exchange, by protoplast fusion and gene cloning, with their unrivalled powers for manipulating genes and genotypes.
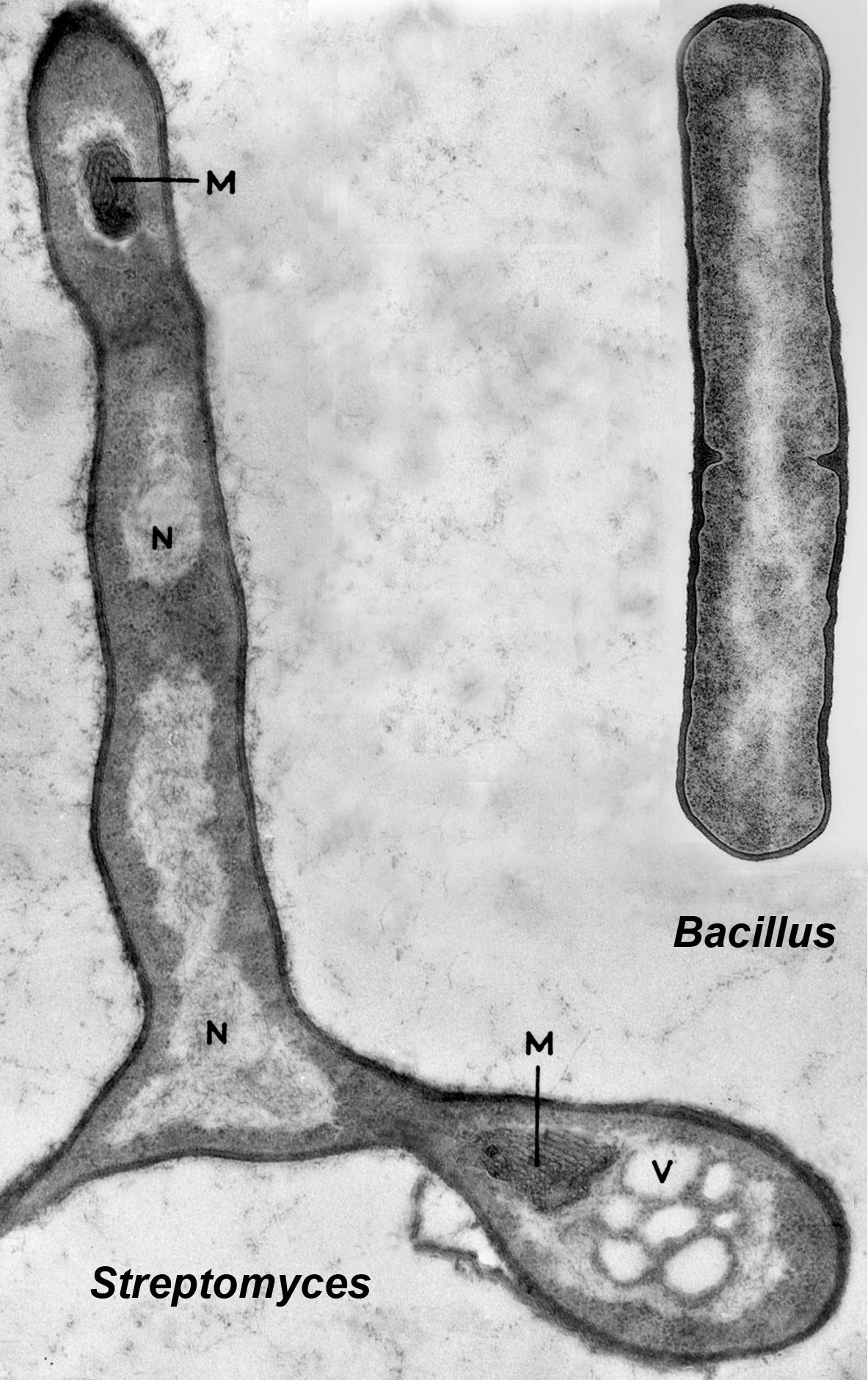
Figure 1. Electron microscope images of a thin section of a germinating spore of Streptomyces coelicolor and of a dividing Bacillus subtilis cell, showing an identical prokaryotic organisation. In both organisms the central pale areas contains the DNA (labelled N in the Streptomyces image) and is not separated by a membrane from the cytoplasm containing the dot-like ribosomes. (M in the Streptomyces image marks regions of intracellular membranes called mesosomes, which are also often seen in Bacillus, while V is a vacuole.) This is a montage of two separate images, provided by Audrey Glauert for Streptomyces and Jeffrey Errington for Bacillus.‘Streptomyces in Nature and Medicine: The Antibiotic Makers’, David A. Hopwood, Oxford University Press, 2007, pp. 62 Reproduced by permission of Oxford University Press.
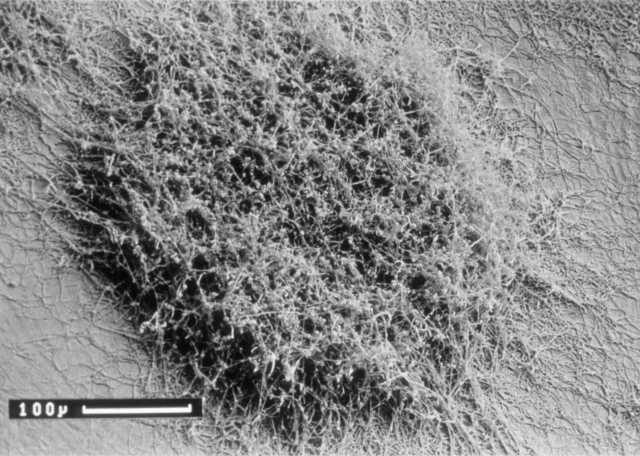
Figure 2. Scanning electron micrograph of a young Streptomyces colony on an agar plate. Note the young vegetative hyphae growing from the edge of the colony and the piled-up aerial mycelium in the centre beginning to segment itself into spores, which are below the resolution of the image. The scale bar is 100 micrometers. Courtesy of Kim Findlay. ‘Streptomyces in Nature and Medicine: The Antibiotic Makers’, David A. Hopwood, Oxford University Press, 2007, pp. 124 Reproduced by permission of Oxford University Press.
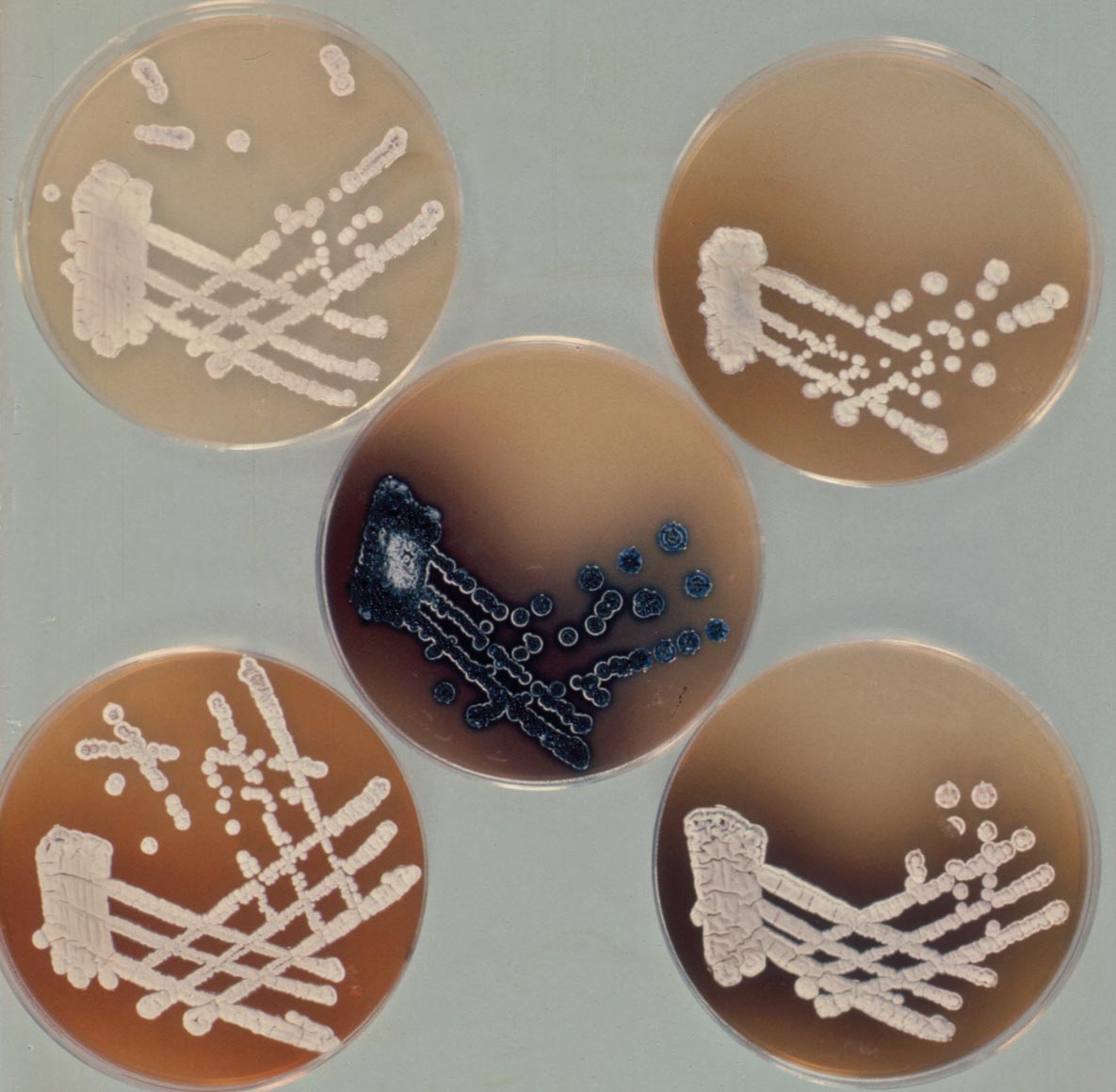
Figure 3. A set of act mutants of Streptomyces coelicolor. The wild type, making the blue antibiotic actinorhodin, is in the middle, surrounded by mutants that fail to produce the blue pigment. The fact that the mutants are making differently pigmented compounds, or none, was a key finding, indicating that they are blocked at different points in the biosynthetic pathway to actinorhodin. The finding that they all mapped to the same short segment of the linkage map revealed a cluster of closely linked genes, a paradigm for all antibiotic biosynthetic pathways. Courtesy of Brian Rudd. Reproduced by permission of Oxford University Press.
